Abstract
Introduction: Venetoclax (ven) in combination with azacitidine (aza) or low-dose cytarabine (LDAC) has demonstrated efficacy for the first-line treatment of acute myeloid leukemia (AML) patients who are deemed unfit for intensive induction chemotherapy. The efficacy of ven-based treatment for relapsed or refractory (r/r) AML has not been prospectively evaluated.
We have used off-label ven-based combinations to treat r/r AML patients and de novo AML in otherwise fit patients to avoid prolonged hospitalization.
The objective of this study was to review the efficacy, toxicity and medication costs associated with ven-based treatments for AML in a Canadian university hospital.
Methods: After local IRB approval, we conducted a retrospective chart review of all patients who received ven-based treatments, outside of a clinical trial, for AML at Hopital Maisonneuve-Rosemont. Supportive care included tumor lysis syndrome (TLS) prophylaxis, antiviral, antifungal and antibacterial prophylaxis.
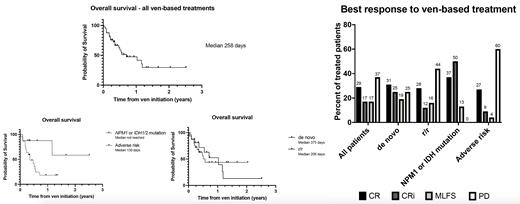
Results: 40 patients received 41 ven-based treatments between November 2017 and July 2021. Most patients had r/r AML (n=25, including 17 in first relapse after allogeneic hematopoietic cell transplantation), while 16 patients had de novo AML (10 deemed fit for intensive chemotherapy). Median age was 62 years old.
Median duration of ven-based treatment was 4 cycles and median follow-up was 140 days after ven initiation. Ven-aza was used for 33 patients. ven-LDAC was used for the first 7 patients. One patient received ven-gilteritinib. Posaconazole was the main antifungal agent used, with ven dose reduced to 70 mg daily (25 patients).
The complete remission (CR) and CR with incomplete hematological recovery (CRi) rate was 46%, higher for de novo vs r/r AML (56% vs 40%). Most patients achieved blast clearance with treatment: CR+CRi+morphologic leukemia-free state (MLFS) rate 63% (75% de novo and 56% r/r). Median overall survival was 258 days (376 days for ven-aza treated patients).
All 8 patients (de novo n=5, r/r n=3) with NPM1 or IDH1/2 mutations achieved blast clearance (CR+CRi 87%), while 22 patients with adverse-risk AML as defined by 2017 ELN risk stratification had a lower yet respectable response rate (CR+CRi 36%).
Ven-LDAC had no activity in advanced disease, with no response for 6 r/r patients who all had adverse-risk AML, and is no longer used in our institution.
The majority of patients were able to receive treatment on an outpatient basis after a brief hospital stay during ven ramp-up. Five patients remained hospitalized for the entire first cycle. Four patients died from infectious causes during the first cycle (2 unfit patients with first line ven-aza, 2 r/r patients with ven-LDAC). Two cases of suspected TLS requiring treatment delays occurred, with no clinical TLS. Venetoclax dose reductions for hematological toxicity were frequently required (51% for all patients, 73% of patients that achieved a response to ven).
The average medication cost per cycle of ven-aza was 4 394 $ CAN and was 6 765 $ CAN per cycle for ven-LDAC.
Conclusion: In this retrospective real-life review, ven-based treatment produced response rates in line with published prospective evidence among de novo AML patients, and a very clinically meaningful response rate for r/r AML, where therapeutic options are limited and outcomes with conventional chemotherapy dismal. The presence of NPM1 or IDH1/2 mutations was predictive of high response rates. Dose reductions were often required for cytopenias but non-hematological toxicities were limited. Although associated with significant cost, ven-aza represents a safe and effective treatment option for r/r AML which can successfully be delivered in an ambulatory setting.
Bouchard: Otsuka: Consultancy; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy. Bambace: AbbVie: Consultancy; Excelthera: Research Funding; Kiadis: Research Funding. Bernard: Kiadis: Research Funding; Excelthera: Research Funding; BMS: Consultancy; Taiho: Consultancy. Hebert: BMS-Celgene: Research Funding. Bergeron: Jazz Pharmaceuticals: Consultancy; Amgen: Consultancy; Servier: Consultancy; BMS: Consultancy; AbbVie: Consultancy; Pfizer: Consultancy.
Venetoclax to treat relapsed or refractory acute myeloid leukemia

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal